GCSE Physics
These are exports from the interactive whiteboard notes made during the lesson. No attempt has been made to edit them. Not all lessons will be published. If you print these you will use a lot of ink!
Search This Blog
Tuesday, 29 April 2014
Wednesday, 2 April 2014
Tuesday, 1 April 2014
Thursday, 27 March 2014
Homework on Gases
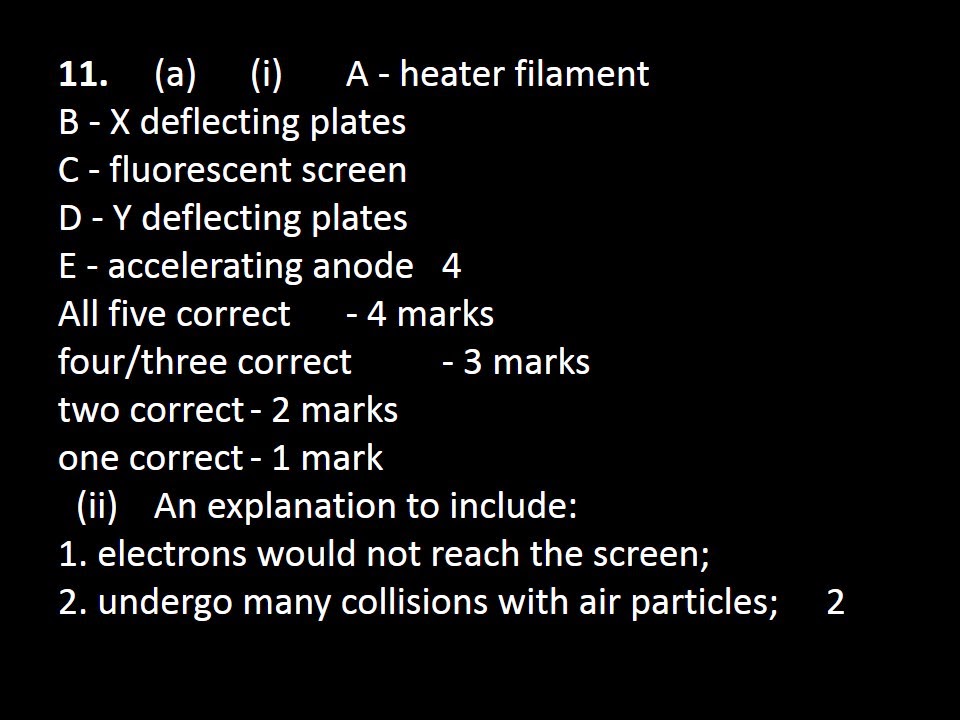
1. (a) An
explanation to include:
1. moving gas particles;
2. hitting container walls;
[Allow bouncing off the sides for 2 marks]
[Ignore vibrating/collisions with other particles] 2
2. hitting container walls;
[Allow bouncing off the sides for 2 marks]
[Ignore vibrating/collisions with other particles] 2
(b) increases;
increases;
stays the same;
stays the same; 4
increases;
stays the same;
stays the same; 4
(c) (i) increases;
in proportion/ 2
in proportion/ 2
(ii) correctly indicated – intercept with
horizontal axis; 1
(iii) zero/minimum; 1
[10]
2. (a) four points plotted correctly;;
4/3 correct - 2 marks
2 correct - 1 mark
straight line drawn; 3
4/3 correct - 2 marks
2 correct - 1 mark
straight line drawn; 3
(b) –260 to –280;;
[Allow an answer between +260 – +280 for 1 mark] 2
[Allow an answer between +260 – +280 for 1 mark] 2
(c) impossible
temperature/this is below absolute zero/pressure is
zero/no movement; 1
zero/no movement; 1
(d) (i) 273; 1
(ii) –196;;
[Allow 77-273 for 1 mark] 2
[Allow 77-273 for 1 mark] 2
[9]
3. (a) (i) (particles)
collide with tyre wall;
Ignore
push/pushing
this produces a force; 2
(ii) more
collisions (/sec); 1
(b) correct conversion into K;
1.72 × 105 = P2; 3
for
2 marks accept any one of
2.08
× 105
temps
swopped over (1.68 × 105)
for
1 mark accept 1.39 × 105
units
if seen must be correct
do not accept 1.7 unless the working is shown
do not accept 1.7 unless the working is shown
[6]
4. (a) (i) particles collide with/hit/bounce off
(container) wall/eq; 1
(ii) An explanation to include:
1. greater volume/space/room;
2. fewer/less collisions (with container); 2
2. fewer/less collisions (with container); 2
(b) (i) increases/eq; 1
(ii) increases/eq; 1
[5]
5. (a) (102
+ 273 =) 375; 1
(b)
|
|
decreases
|
Stays
the same |
increases
but does not double |
doubles
|
|
|
temperature
of gas
|
|
|
|
|
1
|
|
pressure
of gas
|
|
|
|
|
1
|
|
kinetic
energy of particles
|
|
|
|
|
1
|
;;;
(c) collisions; 1
with walls etc; 1
with walls etc; 1
[6]
Subscribe to:
Comments (Atom)