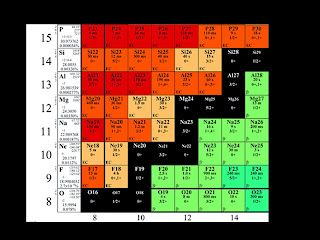
Nuclear
Decay Worksheet
Name the
type of emission:
1.
Uranium-234 to Thorium-230
2.
Thorium-230 to Radium-226
3.
Radium-226 to Radon-222
4. Radon-222
to Polonium-218
5.
Polonium-218 to Lead-214
6. Lead-214
to Bismuth-214
7.
Bismuth-214 to Polonium-214
8.
Polonium-214 to Lead-210
9. Lead-210
to Bismuth-210
10.
Bismuth-210 to Polonium-210
11.
Polonium-210 to Lead-206
Write an
equation for the following elements through the given emission type.
Alpha Decay:
2.
Polonium-214
3.
Polonium-210
4.
Radium-224
5.
Radium-220
6.
Radon-220
7. Radon-219
Beta minus
Decay:
2. Lead-214
3.
Bismuth-210
4.
Bismuth-212
5.
Bismuth-214
- Carbon – 11 11 12C à 11 10X + e+
- Sodium – 22
- potassium-40
- nitrogen-13
- oxygen-15
- fluorine-18
- iodine- 121